Possible Influences of MFRPS on the Planned Adoption and Implementation of the Preventive Controls for Human Food Rule
Kirsten Knopff
Minnesota Department of Agriculture
Food and Feed Safety Division
International Food Protection Training Institute (IFPTI)
2015 Fellow in Applied Science, Law, and Policy: Fellowship in Food Protection
Author Note
Kirsten Knopff, Business and Quality Management Supervisor, Food and Feed Safety Division, Minnesota Department of Agriculture
This research was conducted as part of the International Food Protection Training Institute’s Fellowship in Food Protection, Cohort V.
Correspondence concerning this article should be addressed to Kirsten Knopff, Minnesota Department of Agriculture, Food and Feed Safety Division,
625 Robert Street N, St. Paul, MN 55155
Email: kirsten.knopff@state.mn.us
Abstract
The purpose of this study was to identify if state programs’ level of implementation/conformance with Manufactured Food Regulatory Program Standards (MFRPS) Standard 1 – Regulatory Foundation and Standard 8 – Program Resources influence the state program’s plan to adopt, and its capacity to implement the Food Safety Modernization Act (FSMA) Preventive Controls for Human Food rule (PCHF). The PCHF will have a significant impact on the way that state manufactured food regulatory programs conduct their work. Implementation by state programs of the PCHF may require legislative or rule changes, training, and additional resources. Forty-two programs are working toward implementation and conformance with MFRPS in 40 states. An online survey was conducted of the 42 programs currently enrolled in the MFRPS. State programs may be in partial or full implementation, or partial or full conformance with a Standard, depending on whether required procedures and systems are in place and working effectively. A larger relative percentage of state programs in full implementation, partial conformance, and full conformance, with Standard 1 plan to adopt the PCHF in comparison to state programs at partial implementation. There was no relationship between Standard 8 implementation/conformance and the state program’s capacity for implementation of the PCHF. The author recommends training and guidance for state programs, along with investigating funding mechanisms and resources to help state programs adopt and implement the PCHF. Finally, the MFRPS should be updated to reflect the PCHF, which will require state programs to assess adoption and implementation capacity.
Keywords: Manufactured Food Regulatory Program Standards (MFRPS), Food Safety Modernization Act (FSMA), Preventive Controls for Human Food rule (PCHF), food regulatory standards, state implementation of FSMA, state adoption of FSMA
Background
The Manufactured Food Regulatory Program Standards (MFRPS) establish a uniform foundation for the design and management of state programs responsible for the regulation of food plants (U. S. Food and Drug Administration [FDA], 2013). Since 2007, state programs have enrolled in the MFRPS in different years and are at varying levels of conformance with the MFRPS due to enrollment dates, resources, and agency support. There are ten distinct standards in the MFRPS, but this study limits analysis to Standard 1 – Regulatory Foundation, and Standard 8 – Program Resources. Standard 1 describes the elements of the regulatory foundation used by a state program to regulate food plants, and Standard 8 describes the elements for assessing the resources (staff, equipment, and funding) needed to support a manufactured food regulatory program (FDA, 2013).
Forty-two programs are working toward implementation and conformance with MFRPS in 40 states. (FDA, 2016). Based on draft definitions in the 2016 version of the MFRPS and from U. S. Food and Drug Administration (FDA) audit staff, state programs are assigned a full implementation audit status when procedures and systems are in place, but the state program is unable to demonstrate the procedures and systems are being used. An audit status of full conformance means that a state program is using and can demonstrate the use of procedures and systems required by the Standard. The FDA conducts audits of state program implementation with the Standards every 18, 36, and 60 months. During an audit of a state program, a Standard status of partial or full implementation is assigned, and conformance is determined for each Standard. If a state program is found to have fully implemented all of the standards, the auditors will evaluate the program for full conformance.
The Food Safety Modernization Act (FSMA) was signed into law by President Obama on January 4, 2011 (FDA, 2015a). Since then, the FDA proposed a number of new rules, updated these rules based on public comment, and published several of the new rules. The Final Rule for Preventive Controls for Human Food (PCHF) was published by the FDA in September 2015 in 21 CFR Part 117 (FDA, 2015b). The industry has specific timelines for compliance: between one to three years depending on size and income limits, to comply with the PCHF. During this time, state programs are examining their legal authorities to determine if they have the legal authority necessary to adopt this new rule. Assessments are also being carried out to determine the additional programmatic resources required to conduct preventive control inspections.
The MFRPS have significantly changed many manufactured food state inspection programs since the Standards require written procedures, training and evaluation documentation, and other accountabilities. However, the PCHF will change expectations of manufactured food inspection further in the next few years, due to the new requirements in the rule. Because the PCHF have not yet been implemented, there is a lack of research related to state programs’ intent regarding the adoption of the PCHF rules or if state programs have the capacity to implement the new rules. If state programs do not have the legal authority, capacity, or desire to adopt the preventive control rule, state programs may have the option of using FDA credentials during FDA contract inspections to conduct inspections using the PCHF.
Problem Statement
Whether implementation of and conformance with the MFRPS Standards 1 and 8 effects a state program’s plan to adopt, or the state program’s capacity to implement, the PCHF is not known.
Research Questions
What are the state programs’ current levels of implementation/conformance with MFRPS Standard 1, Regulatory Foundation, and Standard 8, Program Resources?
What are the state programs’ plans to adopt and capacity to implement the PCHF?
Does a state program’s level of conformance with the MFRPS Standard 1 effect the likelihood that a state program will adopt the PCHF?
Does a state program’s level of conformance with the MFRPS Standard 8 effect state programs’ capacity to implement the PCHF?
Methodology
An electronic survey was sent to manufactured food program managers and MFRPS coordinators for the 42 state programs enrolled in the MFRPS as of October 2015. Contacts for state programs were identified using the MFRPS enrollment directory as of October 29, 2015, the Manufactured Food Regulatory Program Alliance (MFRPA) attendee list from the 2015 MFRPA meeting, and the Association of Food and Drug Officials (AFDO) Directory of State and Local Officials (DSLO) as of October 29, 2015. The survey was distributed by email and responses were collected via SurveyMonkey® and/or by email attachment, based on respondent preference. The survey was conducted between October 2015 and December 2015. Survey questions captured general descriptive data for each state program, MFRPS cooperative agreement information, and data related to the state program’s implementation and conformance with Standards 1 and 8 of the MFRPS.
State programs provided the designation of partial implementation, full implementation, and full conformance given during their last audit. Although not an official audit status, for this study an additional status of partial conformance was provided as a response option to identify states that felt they were partially conformant with either Standard 1 or 8. The survey also collected data regarding the state’s plan to adopt the PCHF rules and the capacity of the state program to implement the PCHF. The survey tool did not provide a contextual definition of “capacity” but allowed the respondent to interpret these words based on the respondent’s perception.
Results
Standard 1 Implementation/Conformance and Plan to Adopt the PCHF
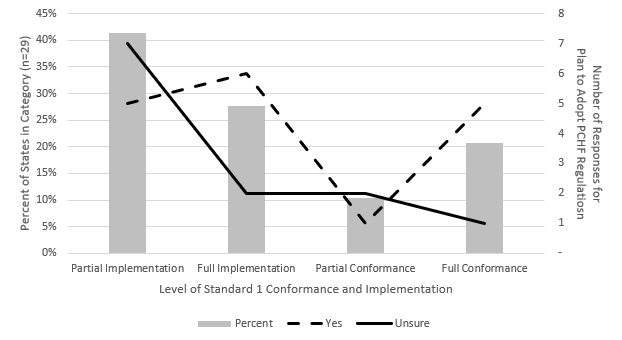
Twenty-nine of the 42 state programs (69%) provided complete answers to the survey. Seventeen of the 29 state programs (59%) responded that they plan to adopt the PCHF. Twelve (41%) replied that they do not know if they will adopt the PCHF. Zero state programs responded that they do not plan to adopt the PCHF at this time. Figure 1 shows the state programs at each level of implementation and conformance with Standard 1 – Regulatory Foundation and how the level corresponds to the state programs’ intent to adopt the PCHF.
State programs in full implementation, partial conformance, and full conformance with Standard 1 plan to adopt the PCHF at a higher rate 12/17 (71%) than state programs currently in partial implementation 5/12 (42%). State programs in full implementation, partial conformance, and full conformance have completed and legally reviewed MFRPS Standard 1 self-assessment, a document that assesses the state’s authority in comparison to the FDA’s authority, and have a written plan/procedure for an annual review to determine equivalency between state and federal regulations at the time of their last FDA MFRPS audit. State programs in partial implementation may not have completed the self-assessment or may not have a written plan for an annual review of regulations at the time of their last audit.
Figure 1. Standard 1: Implementation/Conformance and the Plan to Adopt the PCHF Regulations
Standard 8 Implementation/Conformance and Capability to Implement the PCHF
Nineteen of the 29 respondents (66%) intend to implement the PCHF and conduct inspections using the new rules. Nine respondents (31%) responded that they are unsure if the program will implement the PCHF and conduct inspections using the PC rules. One respondent (3%) did not answer the question.
The survey also asked respondents to consider their state program’s perceived capacity to implement the PCHF. Five of the 29 (17%) respondents believed that their state programs have the capacity to implement the PCHF, 13 (45%) did not think that their state programs have the capacity, and 10 (35%) were unsure if their state programs have the capacity. One (3%) state did not answer this question.
Respondents conveyed the need for additional funding (90%), inspection staff (80%), industry partnerships (55%), equipment (38%), and office space (21%) in order to implement the PCHF. Along with specific resources required, respondents stated the availability of training (38%) and legislative/leadership support of the PCHF (28%) are other possible obstacles to implement PCHF.
Figure 2 shows state programs at each level of implementation and conformance with Standard 8 – Program Resources and how the level corresponds to the perceived capacity of the state program to implement the PCHF regulations. Standard 8 requires state programs to assess the current program resource needs and identify staff, funding, and equipment required to maintain full conformance with each Standard.
Based on survey responses, 2/2 (100%) of state programs in full conformance with Standard 8 are unsure if they have the capacity to implement the PCHF. Of those state programs in partial conformance with Standard 8, 2/7 (29%) believe their programs have adequate capacity to implement the PCHF, while 1/11 (9%) in full implementation responded that their programs have adequate capacity, and 2/9 (22%) of state programs partially implementing Standard 8 responded that their programs have adequate capacity for implementation of the PCHF.
Figure 2. Standard 8: Implementation/Conformance and the Capacity to Implement the PCHF Regulations
Conclusions
The level of implementation/conformance with Standard 1 appears to be related to a state program’s intention to adopt the PCHF based on the higher rate of planned adoption by state programs in full implementation, partial conformance, and full conformance in comparison to state programs in partial implementation. Further research is required to identify if there are other factors, such as enrollment date and overall awareness of requirements, which may correlate with the intent to implement the PCHF. Further research is also required to determine if the annual legal review process required for full conformance with Standard 1 may contribute to a state program’s overall awareness of statutory authorities. Additionally, does the annual review process prompt strategic discussions around adoption of the PCHF in order to maintain regulatory consistency with the FDA.
The level of implementation/conformance with Standard 8 does not appear to correlate with the perceived capacity to implement PCHF. There was no Standard 8 implementation or conformance category where a majority of state programs conveyed that there was capacity for implementation of the PCHF. The variety of responses in each category suggests that most state programs recognize that additional resources will be required to implement the PCHF. State programs also identified the need for additional resources to implement the PCHF such as funding, inspection staff, industry partnerships, equipment, and office space.
Thirty-five percent of respondents replied that they are unsure if their programs have the capacity to implement PCHF, which may suggest that state programs lack an understanding of the resources needed for implementation. Throughout the survey, respondents conveyed their need for additional funding for staff, training, and equipment, along with support from the state legislatures and department heads to adopt and implement the PCHF.
Recommendations
1. Additional research should be conducted to re-examine the issues studied in this research, after the state programs have more information about PCHF adoption and implementation.
2. Outreach, training, and support should be provided related to the adoption of the PCHF such as meetings with legislators, commissioners, and other types of leadership in positions to influence the adoption process.
3. State programs and the FDA should create additional guidance such as estimated PCHF inspection times based on mock inspections, record keeping requirements by the state program, and estimated training time for inspectors to assess the resources required for state program implementation of the PCHF.
4. Funding mechanisms should be created to assist state programs in the adoption and implementation of the PCHF.
5. Resources should be provided to assist state programs in the adoption and implementation of the PCHF.
6. MFRPS should be updated to reflect the new requirements related to the PCHF to help state programs assess conformance with federal regulations and resource assessment.
Acknowledgments
First, I would like to thank all of the state manufactured food programs that replied to my survey and provided thoughtful answers. I also appreciate the FDA Audit team and Office of Partnership staff answering my questions about the Standards. I am thankful for my director, Dr. Benjamin Miller, for the support in pursuing the Fellowship and the exceptional guidance throughout the process. I would like to thank the International Food Protection Training Institute (IFPTI) and all of the IFPTI leadership for the opportunity to participate, learn, and grow in the Fellowship Program. Additionally, I would like to thank my mentor, Steve Steinhoff, and project subject matter expert, Dr. Paul Dezendorf, for helping me and pushing me through this process. Finally, I would like to thank Cohort V and past cohort Fellows for their support and advice. I could not have done this alone and I am truly grateful for all of the help and guidance along the way.
References
U. S. Food and Drug Administration. (2013). Manufactured Food Regulatory Program Standards: September 2013. Retrieved from: http://www.fda.gov/downloads/ForFederalStateandLocalOfficials/PartnershipsContracts/Overview/UCM377447.pdf
U. S. Food and Drug Administration. (2015a). FDA Food Safety Modernization Act (FSMA). Retrieved from: http://www.fda.gov/Food/GuidanceRegulation/FSMA/
U. S. Food and Drug Administration. (2015b). FSMA final rule for Preventive Controls for Human Food. Retrieved from http://www.fda.gov/Food/GuidanceRegulation/FSMA/ucm334115.htm
U. S. Food and Drug Administration. (2016). Manufactured Food Regulatory Program Standards (MFRPS). Retrieved from: http://www.fda.gov/forfederalstateandlocalofficials/programsinitiatives/regulatoryprgmstnds/ucm475064.htm