vILT Radiological Health Inspections Course
IFPTI is excited to announce the development of a new virtual instructor-led course titled Basic Radiological Health Inspections (RH 210).
IFPTI led the design and development of this new course with a group of radiological health subject matter experts from FDA, including experts from the Winchester Engineering Analytical Center (WEAC), which serves as the lead laboratory for medical device and radioanalytical testing.
This new course will be a requirement for FDA Consumer Safety Officers (CSOs) who could potentially conduct inspections or investigations that cover radiation-emitting products, including but not limited to radiological health investigators. Additionally, the course will be available to secondary audiences such as management and compliance officers, recall coordinators, laboratory personnel, and other state/local agency personnel.
The course comprises eight units, which are filled with discussions, activities, videos, graphics, Q & A sessions, knowledge checks, and other interactivities utilizing virtual platform features. Topics include:
the Electronic Product Radiation Control (EPRC) Program
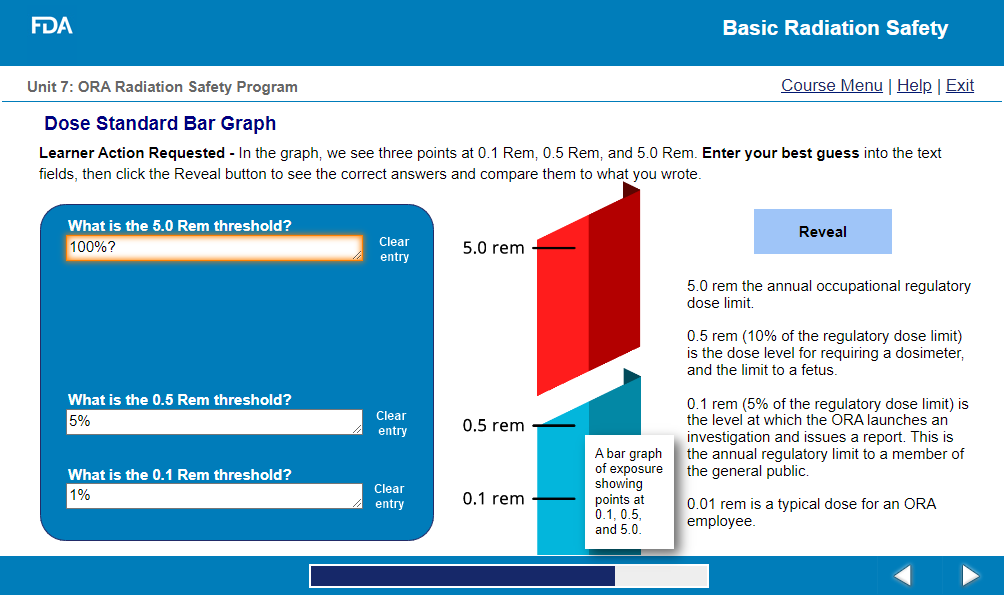
the scientific/technical background of radiological health
manufacturer activities related to reporting/recordkeeping
EPRC inspections
references and resources related to radiological health
the role of WEAC; and
the components of a sunlamp field test.
Note: Development of RH 210 was made possible through Cooperative Agreement 1U18FD007047 between IFPTI and FDA.