Aflatoxin: Occurrence, Prevention, and Gaps in Both Food and Feed Safety in North Carolina
Jennifer L. Godwin
Food and Feed Administrator
North Carolina Department of Agriculture & Consumer Services
International Food Protection Training Institute (IFPTI)
2011 Fellow in Applied Science, Law, and Policy: Fellowship in Food Protection
Abstract
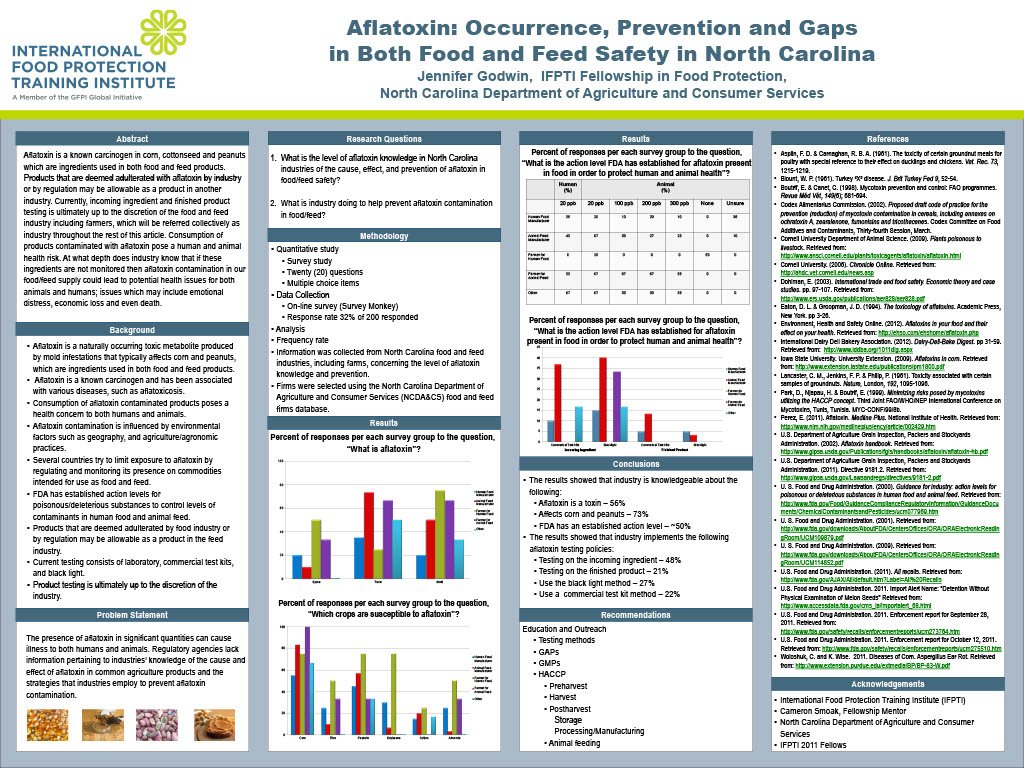
Aflatoxin is a naturally occurring toxic metabolite produced by mold infestations affecting as much as one-quarter of global food and feed crop output. This toxin has been associated with various diseases, such as aflatoxicosis, in livestock, domestic animals, and humans (Dohlman, 2003). To ensure food and feed safety, many countries have adopted regulations to limit exposure to aflatoxin. The primary purpose of this study was to evaluate the industry’s knowledge of aflatoxin in food and feed safety. An online survey was submitted to certain industries in North Carolina to determine their knowledge about the occurrence and possible health effects of aflatoxin, as well as strategies to prevent exposure to this toxin. Less than 50% of respondents knew there was an action level established by the U.S. Food and Drug Administration (FDA) for aflatoxin present in food and feed in order to protect human and animal health. The results showed that 56% of respondents knew that aflatoxin was a toxin, and among those respondents, 73% knew that it affected commodities such as corn and peanuts. The majority of aflatoxin testing, conducted by the industry, is only on the incoming ingredient (48%), and it is conducted primarily by the use of a black light (27%) or commercial test kits (22%). The conclusion of this study is that a majority of the respondents are aware of what aflatoxin is and know about effects on commodities. However, regulation and preventive testing may not be an integral part of industry standards. Ultimately, continuing education on the occurrence of aflatoxin and strategies to prevent exposure to it would help continue to bridge the gap in food and feed safety in North Carolina.
Background
In 1960, an acute hepatotoxic disease in turkeys termed “Turkey X disease” focused the attention of many scientific laboratories on a common problem affecting animals in many areas of the world (Blount, 1961; Lancaster, Jenkins, and Philip, 1961). The dramatic outbreak of the disease, which initially killed more than 100,000 turkeys and was subsequently linked to heavy mortality in ducklings and young pheasants, was shown to be associated with peanut meal in the feed (Asplin and Carnaghan, 1961). An investigation determined that the peanut meal was highly toxic with aflatoxin, a naturally occurring toxic metabolite produced by mold infestations, which demonstrated the seriousness of the problem facing the animal food industry. This case ultimately led to the recognition that aflatoxin is both an economic and a public health problem in many areas of the world (Eaton and Groopman, 1994).
Aflatoxin is a mycotoxin produced by fungi, identified as Aspergillus flavus, which contaminates many commodities, such as corn and corn products, peanuts and peanut products, milk, and tree nuts, which are ingredients used in both food and feed products. The occurrence of aflatoxin contamination is influenced by a wide range of environmental factors, including geography; agricultural/agronomic practices; and the susceptibility of the commodity to the fungi during harvest, storage, and/or processing periods (Environment, Health and Safety Online, 2012). Water stress, high-temperature stress, and insect damage of the host plant are major determining factors in mold infestation and toxin production (Cornell University Department of Animal Science, 2009). The geographical location of North Carolina provides aflatoxin with favorable conditions, such as high moisture and high temperature.
The Food and Agriculture Organization (FAO) of the United Nations estimates that 25 to 50% of the world’s food crops are affected by mycotoxins, with aflatoxin being the most prominent (Boutrif and Canet, 1998). Many countries try to limit exposure by regulating and monitoring aflatoxin presence in commodities intended for use as food and feed. Mycotoxins are considered unavoidable contaminants of food and feed. Therefore, to help prevent aflatoxin ingestion, the U.S. Food and Drug Administration (FDA) has established action levels for poisonous/deleterious substances to control levels of contaminants in human food and animal feed in the document Guidance for Industry: Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed (U.S. Food and Drug Administration, 2000). Action levels are established based on the unavoidability of the poisonous/deleterious substance and represent limits at or above which the FDA will take legal action to remove products from the market. The action level for aflatoxin in food and milk is 20 ppb and 0.5 ppb, respectively, and up to 300 ppb in animal feed.
Several methods are currently being used to test for the presence of aflatoxin, such as analytical laboratories, commercial test kits, and black light tests (Iowa State University, 2009). Analytical laboratories are highly accurate and quantitative and use one of several procedures, such as thin-layer chromatography, gas chromatography, or mass spectroscopy, to determine aflatoxin levels. Commercial test kits using immunoassay or ELISA techniques, which are based on the detection of specific proteins found in aflatoxins using antibodies, are available for on-site tests for aflatoxin. The black light (also called ultraviolet light) test is a visual inspection for the presence of a greenish-gold fluorescence under light at a wavelength of 365 nm (nanometers). Because aflatoxin does not occur uniformly through a commodity and is usually localized in a small area, the best approach is to make a composite sample consisting of subsamples from every part of a load, bin, or unit of corn. The North Carolina Department of Agriculture and Consumer Services (NCDACS) requires testing for aflatoxin in corn products prior to use in products for human consumption, as described in the North Carolina Administrative Code (NCAC) (02 NCAC 09J. 0101). However, food products that are deemed adulterated by industry or regulation may be allowable as a feed product and therefore diverted into feed products. These products may then be consumed by pets and livestock.
Exposure to aflatoxin is difficult to avoid, since fungal growth in commodities is not easy to prevent. Aflatoxin has been associated with various diseases, such as aflatoxicosis (a hepatic disease) in livestock, domestic animals, and humans. Susceptibility to aflatoxicosis varies depending on age, sex, and nutrition of both humans and animals. In developed countries, food and feed products contaminated with specific levels of aflatoxin are not permitted. However, concern still remains regarding the possible adverse affects from long-term exposure to low levels of aflatoxins in the food supply. In July 2011, there were two Class II recalls due to elevated levels of aflatoxin in peanut butter (U.S. Food and Drug Administration, 2011). In 2001 and 2009, the FDA cited a company for shipping peanuts contaminated with aflatoxin (U.S. Food and Drug Administration, 2001; U.S. Food and Drug Administration, 2009).
In animals, aflatoxin can cause liver damage, decreased milk and egg production, gastrointestinal dysfunction, reduced reproductivity, and reduced feed utilization and efficiency. According to the FDA Recall List, there were pet food recalls due to aflatoxin in 2005 and 2010, and, most recently, there were five recalls in December 2011 (U.S. Food and Drug Administration, 2011). In 2005, more than 100 canine deaths and at least one feline fatality were linked to pet food contaminated with aflatoxin, according to Cornell University veterinarians (Cornell University, 2006).
Aflatoxin is a particular problem in underdeveloped countries, which can cause concern over imported products. According to the FDA, melon seeds from the Sudan have been on “Detention Without Physical Examination” since 1982 due to violative levels of aflatoxin. Shipments continue to be offered for entry and refused due to the presence of aflatoxin (U.S. Food and Drug Administration, 2011). As the ethnic population in the United States continues to grow, so does the popularity of imported products. According to the International Dairy Deli Bakery Association (IDDBA), the ethnic food segment continues to grow due to a combination of factors, such as an increase in immigrants, more international travel, and a rising interest in cooking and cooking shows that inspire cooking of traditional and nontraditional recipes (International Dairy Deli Bakery Association, 2012).
Aflatoxin is considered an unavoidable contaminant of food and feed. For this reason, action levels were established at which the FDA will take legal action to remove products from the market. Aflatoxin contamination of food and feed poses both human and animal health concerns. The geographical location of North Carolina provides aflatoxin with favorable growing conditions, such as high moisture and high temperature. Therefore, people who may use susceptible commodities need to understand the occurrence of aflatoxin, its possible health effects, and strategies to prevent exposure to it through food and feed.
Problem Statement
The presence of aflatoxin in significant quantities can cause illnesses in both humans and animals. Ingredients and finished feed deemed adulterated with aflatoxin by one industry group or by regulation may potentially enter another industry group. Regulatory agencies lack information pertaining to industries’ knowledge of the cause and effect of aflatoxin in common agricultural products and the strategies that industries employ to prevent aflatoxin contamination.
Research Questions
This study examined industries’ knowledge of aflatoxin in common ingredients, such as corn and peanuts, used in North Carolina food and feed manufacturing.
1. What is the level of aflatoxin knowledge in the industry of the causes and effects of aflatoxin contamination in the food and feed supply?
2. What is the industry doing to help prevent aflatoxin contamination of food and feed?
Methodology
A 20-question online survey was submitted to the North Carolina food and feed industry, including farmers, to collect information concerning the industry’s knowledge of aflatoxin and strategies to prevent exposure to this toxin. The survey was sent to approximately 200 North Carolina firms selected from the NCDACS, Food and Drug Protection Division, Food and Feed firms database based on industry codes that identify (?) the firm type. Firm types, such as bakeries, flour mills, dairy farms, peanut processors, cereal/breakfast food manufacturers, and animal feed manufacturers, were selected based on their potential use of commodities that are susceptible to aflatoxin contamination in food and feed manufacturing. The survey identified the establishment size and primary purpose of the firms (human food manufacturer, animal feed manufacturer, or crop farmer). Next, questions about the identity of aflatoxin, i occurrence, regulation, and possible effects on human and animal health were used to help gather information on the firms’ knowledge of aflatoxin. Finally, the survey explored the firms’ policy on testing for aflatoxin in susceptible commodities.
Results
The survey was designed to capture information regarding each firm’s establishment size and purpose, knowledge of aflatoxin occurrence, policy on aflatoxin testing, and opinion of aflatoxin testing. Of the respondents, 48% identified their primary purpose as animal feed manufacturers, 32% as human food manufacturers, and 10% or less as farmers for human or animal consumption and other types of firms that handle commodities.
The results showed that 56% of those who responded knew that aflatoxin was a toxin, and among those respondents, 73% knew that aflatoxin affected commodities such as corn and peanuts. Approximately 50% knew that the FDA has established an action level for aflatoxin present in food and feed in order to protect human and animal health. The survey identified each firm’s policy on testing for aflatoxin in susceptible commodities as well as in the finished product. Of those who responded, 48% tested aflatoxin on incoming ingredients only, and no more than 21% conducted aflatoxin testing on the finished product. The results showed that the primary means of testing for aflatoxin were a form of the immunoassay technique (22%) and the use of an ultraviolet lamp or black light (27%). A black light is often used as an initial screen to detect aflatoxin contamination. However, this method is strictly a presumptive test and does not confirm the presence of aflatoxin; only a chemical analysis can verify the presence of aflatoxin (Woloshuk and Wise, 2011). The U.S. Department of Agriculture Grain Inspection, Packers and Stockyards Administration (GIPSA) under Directive 9181.2 has implemented a program to verify the performance of rapid commercial tests for mycotoxins in grains (U.S. Department of Agriculture, 2011). According to the GIPSA Aflatoxin Handbook, there are several approved commercial methods for testing aflatoxin (U.S. Department of Agriculture, 2002).
Conclusions
These research findings suggest that gaps exist in North Carolina’s food and feed industry regarding the occurrence of aflatoxin, possible health effects, regulation, and strategies to prevent exposure. This project found that the industry has awareness of aflatoxin. However, aflatoxin testing is conducted on a limited basis throughout the industry. The testing of ingredients and finished products for aflatoxin contamination is an area of great concern. The survey results showed that a popular testing method is the use of a black light, which is strictly a presumptive test and does not confirm the presence of aflatoxin. Only a chemical analysis can verify the presence of aflatoxin. If ingredients are not monitored, there is the risk of aflatoxin contamination in our food and feed supply, which could lead to potential health issues for both animals and humans. These issues lead to emotional distress, loss of consumer confidence, economic loss, and even death. Risk, such as aflatoxin contamination, is an everyday possibility in business. Those companies that take a proactive approach to risk management often put themselves in a better position to succeed.
Recommendations
As the U.S. strives to build an integrated food and feed safety system, the importance of educational outreach is imperative. One method to help reduce potential health risks and economic losses associated with aflatoxin is to increase awareness among food and feed producers of practices that would minimize aflatoxin contamination and to encourage the adoption of process-based guidelines, such as good agricultural practices (GAPs) and good manufacturing practices (GMPs) (Dohlman, 2003). A Codex Committee on Food Additives and Contaminants (CCFAC) report recommended that GAPs and GMPs be used to establish formal hazard analysis and critical control point (HACCP) food safety systems to identify, monitor, and control mycotoxin risks along the entire food production chain (Codex Alimentarius Commission, 2002). Park et al. (1999) suggested steps to lower mycotoxin contamination that can be taken at the following four stages of food production: preharvest, harvest, postharvest (storage and processing/manufacturing), and animal feeding. For example, at the postharvest processing/manufacturing stage, all susceptible ingredients for aflatoxin should be tested. Incoming ingredient and finished product testing helps ensure that food and feed safety controls are in place. Education and outreach about the identity, occurrence, regulation, and effects of aflatoxin on human and animal health are necessary to promote awareness of this common—but potentially deadly—substance.
Acknowledgements
I would like to extend my sincere appreciation to Cameron Smoak, my mentor in the International Food Protection Training Institute (IFPTI) Fellowship Program for the support, guidance, and understanding throughout the Fellowship Program. I would also like to thank IFPTI for providing an invaluable education and professional experience and my director, Dan Ragan, and the NCDACS for providing the time and support to participate in this experience. I would also like to thank the other Fellows and my coworkers for their support throughout this process. I especially want to thank my husband and son for their unending support, which allowed me to complete the Fellowship program.
Corresponding Author
Jennifer Godwin, North Carolina Department of Agriculture & Consumer Services
Email: jennifer.godwin@ncagr.gov
References
Asplin, F. D. and Carnaghan, R. B. A. (1961). The toxicity of certain groundnut meals for poultry with special reference to their effect on ducklings and chickens. Vet. Rec. 73, 1215-1219.
Blount, W. P. (1961). Turkey “X” disease. J. Brit. Turkey Fed, 9, 52-54.
Boutrif, E. and Canet, C.. (1998). Mycotoxin prevention and control:
FAO programmes. Revue Méd. Vét., 149(6), 681-694.
Codex Alimentarius Commission. (2002, March). “Proposed Draft Code of Practice for the Prevention (Reduction) of Mycotoxin Contamination in Cereals, Including Annexes on Ochratoxin A, Zearalenone, Fumonisins and Tricothecenes.” Codex Committee on Food Additives and Contaminants, Thirty-fourth Session, March.
Cornell University Department of Animal Science. (2009). Aflatoxins: Occurrence and health risks. Retrieved from
http://www.ansci.cornell.edu/plants/toxicagents/aflatoxin/aflatoxin.html
Cornell University. (2006). Chronicle Online. Retrieved from
http://ahdc.vet.cornell.edu/news.asp
Dohlman, E. (2003). Mycotoxin hazards and regulations: Impacts on food and animal feed crop trade. In J. C. Buzby (Ed.), International trade and food safety: Economic theory and case studies (pp. 97-107). U.S. Department of Agriculture. Retrieved from
http://www.ers.usda.gov/publications/aer828/aer828.pdf
Eaton, D. L. and Groopman, J. D.. (1994). The Toxicology of Aflatoxins. Academic Press, New York. pp 3-26.
Environment, Health and Safety Online. (2012). Aflatoxins in your Food—and their effect on your health. Retrieved from
http://ehso.com/ehshome/aflatoxin.php
International Dairy-Deli-Bakery Association. (2011, October). What’s in Store 2012. Consumer Lifestyles. International Dairy-Deli-Bake Association, pp. 31-37. Retrieved from
http://www.iddba.org/pdfs/wis/wis12sample.pdf
Iowa State University, University Extension. (2009). Aflatoxins in corn.
Retrieved from
http://www.extension.iastate.edu/publications/pm1800.pdf
Lancaster, C. M., Jenkins, F. P., and Philip, P. (1961). Toxicity associated with certain samples of groundnuts. Nature,192, 1095-1096.
Park, D., Njapau, H., and Boutrif, E. (1999). Minimizing risks posed by mycotoxins utilizing the HACCP concept. Third Joint FAO/WHO/NEP International Conference on Mycotoxins, Tunis, Tunisia. MYC-CONF/99/8b.
North Carolina Administrative Code. Title 02. Agriculture and Consumer Services. Chapter 09. Food and Drug Protection. SubChapter J. Testing for aflatoxin in cornmeal. Retrieved from
http://ncrules.state.nc.us/ncac/title%2002%20-%20agriculture%20and%20consumer%20services/chapter%2009%20-%20food%20and%20drug%20protection/subchapter%20j/subchapter%20j%20rules.html
U.S. Department of Agriculture Grain Inspection, Packers and Stockyards Administration. (2002). Aflatoxin handbook. Retrieved from
http://www.gipsa.usda.gov/Publications/fgis/handbooks/aflatoxin/aflatoxin-hb.pdf
U.S. Department of Agriculture Grain Inspection, Packers and Stockyards Administration. (2011). Directive 9181.2. Retrieved from
http://www.gipsa.usda.gov/Lawsandregs/directives/9181-2.pdf
U.S. Food and Drug Administration. (2000). Guidance for industry: Action levels for poisonous or deleterious substances in human food and animal feed.
Retrieved from
http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/ChemicalContaminantsandPesticides/ucm077969.htm
U.S. Food and Drug Administration. (2001). Retrieved from
http://www.fda.gov/downloads/AboutFDA/CentersOffices/ORA/ORAElectronicReadingRoom/UCM109879.pdf
U.S. Food and Drug Administration. (2009). Retrieved from
http://www.fda.gov/downloads/AboutFDA/CentersOffices/ORA/ORAElectronicReadingRoom/UCM114852.pdf
U.S. Food and Drug Administration. (2011). All recalls. Retrieved from
http://www.fda.gov/AJAX/All/default.htm?Label=All%20Recalls
U.S. Food and Drug Administration. (2011). Detention without physical examination of melon seeds. Retrieved from
http://www.accessdata.fda.gov/cms_ia/importalert_68.html
U.S. Food and Drug Administration. (2011). Enforcement report for September 28, 2011. Retrieved from
http://www.fda.gov/safety/recalls/enforcementreports/ucm273764.htm
U.S. Food and Drug Administration. (2011). Enforcement report for October 12, 2011.
Retrieved from
http://www.fda.gov/safety/recalls/enforcementreports/ucm275510.htm
Woloshuk, C. and Wise, K. (2011). Diseases of corn: Aspergillus ear rot.
Purdue Extension. Retrieved from
http://www.extension.purdue.edu/extmedia/BP/BP-83-W.pdf